Background: Fit patients (pts) with mantle cell lymphoma (MCL) are commonly treated with immunochemotherapy and consolidative high-dose therapy + stem cell rescue (cHDT/SCR), yet this approach has not demonstrated an overall survival (OS) benefit in a randomized trial. Outcomes for pts with high-risk MCL (TP53 aberrancy, high proliferation index, blastic histology) after cHDT/SCR are poor, and not all pts with MCL are eligible for this approach.
Methods: We conducted a phase II study of sequential immunochemotherapy incorporating lenalidomide enriching for pts with high-risk disease features (defined as blastoid/pleomorphic histology and/or Ki67 >=30%). The three phases of tx were: 1) lenalidomide (15 mg daily, days 1-14) plus R-CHOP for four 21-day cycles; 2) R-HiDAC for 2 cycles (initially age-based cytarabine 1-3 g/m2; 3 g/m2 dose removed after 16 pts due to hematologic toxicity); and 3) rituximab monthly plus lenalidomide (15 mg daily) for 6 months (mos).
Eligibility requirements were untreated stage II-IV MCL, KPS ≥70%, and adequate organ function; we sought ≥2/3 high-risk pts. We performed MRD testing on peripheral blood (cellular DNA) using the clonoSEQ Assay (Adaptive Biotechnologies). We obtained PET/CT and MRD testing after each phase of treatment and also MRD evaluation at 6 mos post-rituximab + lenalidomide maintenance. The primary endpoint was the rate of 3-yr progression-free survival (PFS), (acceptable PFS ≥75%, unacceptable ≤60%, based on desired proportion of high-risk pts).
Results: Among 49 pts enrolled, 47 were evaluable for PFS (1 had progressive disease (PD) and 1 had toxicity during len-R-CHOP). Characteristics for 47 evaluable pts are shown in Table 1: 64% were high-risk and 18% had TP53 mutation. 45 completed maintenance (1 had PD during R-HiDAC and 1 withdrew to pursue cHDT/SCR) and 43 achieved complete response (CR), 1 stable disease, and 1 PD at end of treatment (EoT), yielding overall response rate of 91%, all CR (Figure 1). With a median follow-up of 2.8 yrs among survivors, the 3-yr PFS was 64% (95 CI 50, 82) and OS 85% (95 CI 74, 99). Three-yr PFS differed by TP53 status (14% mut vs. 85% wt, P < 0.0001, Table 2). Of 4 pts with PD, 3 had TP53 mutation and 1 had an unknown mutation status. Among TP53 wt pts, there was no significant difference in outcomes by risk (Table 2).
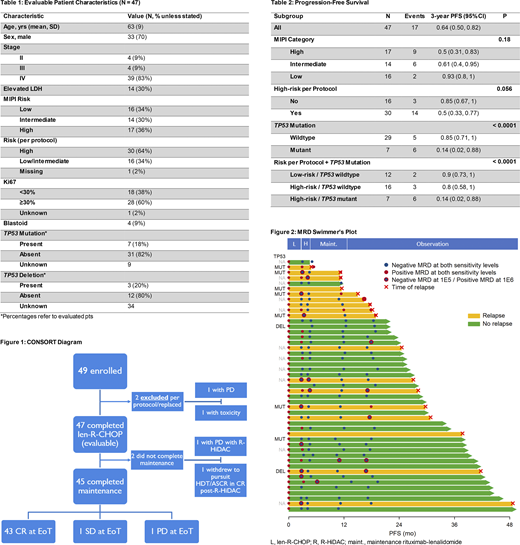
MRD results were not obtained in 4 pts. Among 45 pts with MRD results, tumor clonal characterization for MRD evaluation was successful in 87% (39/45). MRD results are shown in Figure 2.
Examining the initial phase of treatment (len-R-CHOP and R-HiDAC), among 37 pts with results at 1x10-5 sensitivity (1E5) following len-RCHOP, a substantial proportion (32%, 12/37 pts) remained MRD+ and 11 of 12 MRD+ pts post len-RCHOP converted to MRD- following R-HiDAC. At 1x10-6 sensitivity (1E6) following R-HiDAC, 5/20 pts were MRD+, and among responding pts, shorter median PFS was observed in MRD+ versus MRD- pts (23.1 mos vs. NR, P = 0.03).
Examining the final phase of treatment (rituximab + lenalidomide maintenance) and observation period, among 37 pts with MRD results at 1E5 at EoT, 4 were MRD+, 2 of which were simultaneous (within 2 weeks of testing) with relapse; the remaining two MRD+ pts had median PFS 4.9 mos versus 37.4 mos for the 32 non-relapsed MRD- pts (P < 0.001). At 1E6, 6 pts who were MRD- at EoT converted to MRD+ after 6 mos of observation. MRD status at 1E6 at 6-mos post-EOT correlated with PFS: among 20 non-relapsed pts (6 MRD+, 14 MRD-), median PFS was 30.8 mos for MRD- versus 13.2 mos for MRD+ (P = 0.02).
Conclusions: In a novel approach of sequential immunochemotherapy plus lenalidomide enrolling majority high-risk pts, outcomes for TP53-mutant pts were poor and we did not reach our primary endpoint of 3-yr PFS ≥75%. Among TP53-wt pts, this treatment program was highly effective even among pts with elevated Ki-67 (>=30%) and was associated with a high response rate, a 3-yr rate of PFS of 85%, and a high rate of MRD- at EoT.
A substantial proportion of pts converted to MRD- after receipt of R-HiDAC, highlighting the efficacy of cytarabine in MCL. There was a high rate of MRD- after induction chemoimmunotherapy (Len-R-CHOP + R-HiDAC) at 1E5 (97%) and at 1E6 (80%), and the latter predicted remission duration. Several pts converted from MRD- to MRD+ at 6-mos post-EOT and eventually relapsed, suggesting that a more prolonged period of maintenance may be beneficial. Finally, MRD at 1E6 at 6 mos following EoT predicted response duration.
Batlevi:Life Sci, GLG, Juno/Celgene, Seattle Genetics, Kite: Consultancy; Janssen, Novartis, Epizyme, Xynomics, Bayer, Autolus, Roche/Genentech: Research Funding. Dogan:National Cancer Institute: Research Funding; EUSA Pharma: Consultancy; Takeda: Consultancy; Seattle Genetics: Consultancy; Corvus Pharmaceuticals: Consultancy; Physicians Education Resource: Consultancy; Roche: Consultancy, Research Funding; AbbVie: Consultancy. Drullinsky:Novartis: Research Funding; Roche: Research Funding. Gerecitano:Janssen: Current Employment. Hamlin:Portola Pharmaceutics: Consultancy; J&J Pharmaceuticals: Research Funding; Juno Therapeutics: Consultancy; Celgene: Consultancy; Incyte: Research Funding; Molecular Templates: Research Funding; Portola: Research Funding; Karyopharm: Consultancy. Ho:Invivoscribe, Inc.: Honoraria. Jacob:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company. Matasar:Rocket Medical: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Takeda: Consultancy, Honoraria; GlaxoSmithKline: Honoraria, Research Funding; IGM Biosciences: Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Immunovaccine Technologies: Honoraria, Research Funding; Merck: Consultancy; Bayer: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Teva: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Research Funding. Moskowitz:Incyte: Research Funding; Imbrium Therapeutics, L.P.: Consultancy; Seattle Genetics: Consultancy; Miragen Therapeutics: Consultancy; Merck: Consultancy; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Merck: Research Funding. Mullins:Adaptive Biotechnologies: Current Employment, Other: shareholder. Straus:Elsevier: Membership on an entity's Board of Directors or advisory committees, Other: CME writer; NY Lymphoma Rounds: Consultancy; Imedex, Inc.: Speakers Bureau; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Targeted Oncology: Consultancy, Speakers Bureau; ASH: Other: Conference in December 2019 on HL to other physicians during ASH; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Speakers Bureau; Takeda Pharmaceuticals: Research Funding, Speakers Bureau. Younes:BioPath: Consultancy; Daiichi Sankyo: Consultancy; Takeda: Consultancy; Novartis: Consultancy; AstraZeneca: Current Employment; BMS: Consultancy; Curis: Consultancy; Epizyme: Consultancy; HCM: Consultancy; Janssen: Consultancy. Zelenetz:Amgen: Consultancy; Celgene: Research Funding; Genentech/Roche: Consultancy; Sandoz: Research Funding; Novartis: Consultancy; Janssen: Consultancy; Adaptive Biotechnology: Consultancy; Celgene: Consultancy; Gilead: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; MorphoSys: Research Funding; MEI Pharma: Research Funding; Roche: Research Funding. Kumar:Celgene: Honoraria, Other: Honoraria for Advisory Board; Astra Zeneca: Honoraria, Other: Honoraria for Advisory Board; Celgene: Research Funding; Pharmacyclics: Research Funding; Adaptive Biotechnologies,: Research Funding; AbbVie: Research Funding; Seattle Genetics: Research Funding; Kite Pharmaceuticals: Honoraria, Other: Honoraria for Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal